An efficient nutrient medium for asymbiotic seed germination and in vitro plant generation of Vanda tessellata (Roxb.) Hook. ex G. Don
Published 2025-03-10
Keywords
- Banana,
- charcoal,
- genetic stability,
- micropropagation,
- protocorm
- RAPD analysis,
- seedling development ...More
How to Cite
Copyright (c) 2024 Dipak Kumar Kar, Purnima Paramanik, Nirmalya Banerjee, Subrata Raha

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
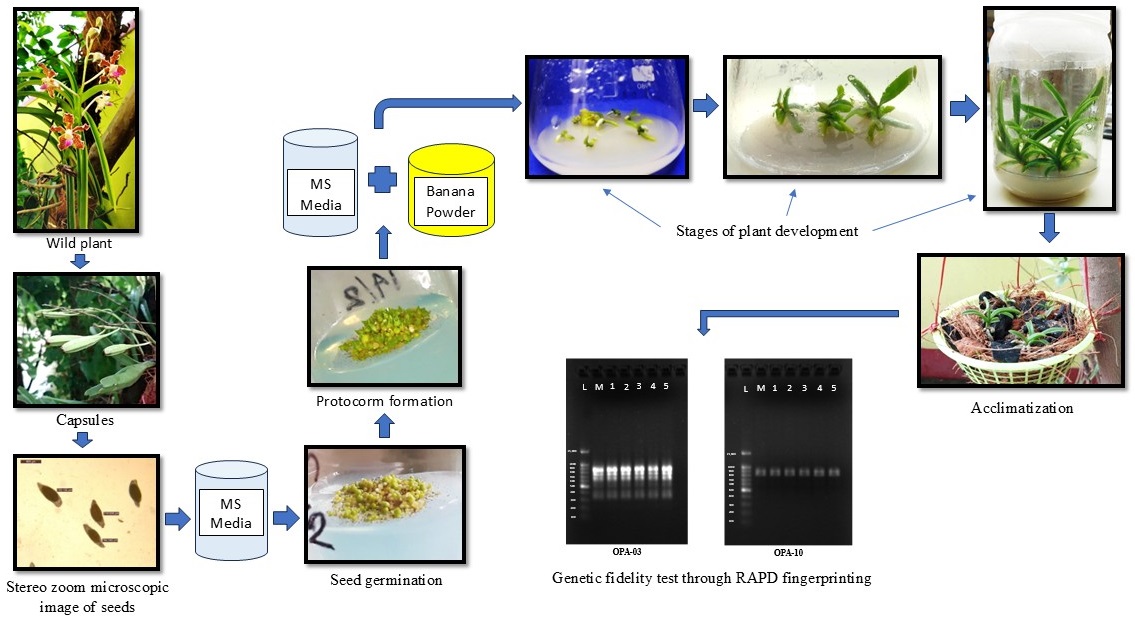
Vanda tessellata (Roxb.) Hook. ex G. Don is an epiphytically grown orchid well-known for its excellent floral value and therapeutic qualities. The present investigation deals with a study of asymbiotic seed germination and large-scale in vitro plant generation of Vanda tessellata by using three different basal media (MS, KC, and VW) and two supplements, charcoal and banana. Of these three media used for seed germination, MS (Murashige and Skoog) gave the best response, followed by KC (Knudson C) and VW (Vacin and Went). MS medium took less time to germinate seeds and maximum protocorm formation was also observed. MS medium with banana powder (15,000 mg/l) showed the best result for developing seedlings from protocorm and maximum growth of leaves and roots of the seedlings. Propagation through secondary protocorm formation was highest in MS media with charcoal (1000 mg/g). In vitro-grown plants were successfully acclimatized with an 89.4% survival rate. According to a random amplified polymorphic DNA (RAPD) analysis, the in vitro generated plants were clone copies of their parent plant and did not exhibit variations. These findings validated the most trustworthy techniques, which can also be applied for to large-scale medicinal Vanda tessellata plant production at the commercial level.
References
- AKTAR S., NASIRUDDIN K.M., HOSSAIN K., 2008 - Effects of different media and organic additives interaction on in vitro regeneration of Dendrobium orchid. - J. Agric. Rural Devel., 6: 69-74.
- BHADRA S.K., 1999 - Development of in vitro micropropagation techniques in some orchid species of Bangladesh. - M.Sc. Dissertation, Dept. of Botany, Chittagong University, Bangladesh.
- CHAUHAN N.S., 1999 - Medicinal and aromatic plants of Himachal Pradesh. - Indus. Publishing Company, New Dehli, India, pp. 632.
- GUTIERREZ R.M.P., 2010 - Orchids: A review of uses in traditional medicine, its phytochemistry and pharmacology. - J. Med. Plants Res., 4(8): 592-638.
- HUSSAIN Z., TYAGI R.K., SHARMA R., AGRAWAL A., 2008 - Genetic diversity in in vitro-conserved germplasm of Curcuma L. as revealed by RAPD markers. - Biol. Plant, 52: 627-633.
- IRSHAD M., RIZWAN H.M., DEBNATH B., ANWAR M., LI M., LIU S., HE B., QIU D., 2018 - Ascorbic acid controls lethal browning and pluronic F-68 promotes high-frequency multiple shoot regeneration from cotyledonary node explant of okra (Abelmoschus esculentus L.). - HortSci., 53(2): 183-190.
- ISLAM M.O., ISLAM M.S., SALEH M.A., 2015 - Effect of banana extract on growth and development of protocorm like bodies in Dendrobium sp. orchid. -Scientific J. Krishi Found., 13(1): 101-108.
- KAUR S., BHUTANI K.K., 2009 - In vitro propagation of Vanda testacea (Lindl.) Reichb. f. - A rare orchid of high medicinal value. - Plant Tissue Cult. Biotech., 19(1): 1-7.
- KAWIAK A., LOJKOWSKA E., 2004 - Application of RAPD in the determination of genetic fidelity in micropropagated Drosera planlets. - In Vitro cell. Dev. Biol. Plant., 40: 592-595.
- KIM D.H., KANG K.W., ENKHTAIVAN G., JAN U., SIVANESAN L., 2019 - Impact of activated charcoal, culture medium strength and thidiazuron on non-symbiotic in vitro seed germination of Pecteilis radiata (Thunb.) Raf. - South Afr. J. Bot., 124: 144-150.
- KUNDSON L., 1946 - A new nutrient solution for the germination of orchid seeds. - Amer. Orchid Soc. Bull., 15: 214-217
- MADHAVI M., SHANKAR P.C., 2019 - Effect of different growth additives on seed germination of Vanda tessellata (Roxb.) Hook. Ex. G. Don. - A medicinal orchid. - J. Orchid Soc. India, 33: 105-112.
- MAGRINI S., DEVITIS M., 2017 - In vitro reproduction of three Limodorum species (Orchidaceae): impacts of scarification methods and nitrogen sources on mature seed germination and seedling development. - Plant Biosystems, 151: 419-428.
- MEZIANI R., JAITI F., MAZRI M.A., HASSANI A., BEN SALEM S., ANJARNE M., AIT CHITT M., ALEM C., 2016 - Organogenesis of Phoenix dactylifera L. cv. Mejhoul: Influences of natural and synthetic compounds on tissue browning, and analysis of protein concentrations and peroxidase activity in explants. - Scientia Hortic., 204: 145-152.
- MINEA M., PILUEK C., MENAKANI T.A., TANTIWIWAT S., 2004 - A study on seed germination and seedling development of Spathoglottis kimballiana. - Agric. Nat. Resources, 38 (2): 141-156.
- MITRA S., 2021 - Diversity of the orchids flora of West Bengal, India. - Plant archives, 21(2): 740-756.
- MITTAL P., DEVI R., GOSAL S.S., 2016 - Effect of genotypes and activated charcoal on high frequency in vitro plant regeneration in sugarcane. - Indian J. Biotech., 15: 261-265.
- MURASHIGE T., SKOOG F., 1962 - A revised medium for rapid growth and bio assays with tobacco tissue cultures. - Physiol. Plant., 15(3): 473-497.
- PAN M.J., VAN STADEN J., 1998 - The use of charcoal in in vitro culture-a review. - Plant Growth Regulation, 26: 155-163.
- PARAMANIK M., MAHATO A., RAHA S., 2020 - Orchids in Purulia District, West Bengal. - J. Botan. Soc. Bengal, 74(2): 124-131.
- PARAMANIK P., KAR D.K., RAHA S., 2021 - A review on asymbiotic seed germination in orchids through plant tissue culture. - J. Scientific Enquiry, 1(1): 1-7.
- PIERIK R.L.M., 1987 - In vitro culture of higher plants. - Martinus Higoff Publ, The Netherlands, pp. 344.
- PRADHAN S., PAUDEL Y.P., QIN W., PANT B., 2023 - Genetic fidelity assessment of wild and tissue cultured regenerants of a threatened orchid, Cymbidium aloifolium using molecular markers. - Plant Gene, 34: 100418.
- QAMAR S., SHAIKH A., 2018 - Therapeutic potentials and compositional changes of valuable compounds from banana. A review. - Trends Food Sci. Technol., 79: 1-9.
- RANI D., DANTU P.K., 2016 - Sustained shoot multiplication and method for overcoming in vitro browning in medicinally important plant, Piper chaba Hunt. - Proc. Nat. Academy Sci., India- Section B: Biological Sciences, 86(2): 407-413.
- RAWAT J.M., RAWAT B., AGNIHOTRI R.K., CHANDRA A., NAUTIYAL S., 2013 - In vitro propagation, genetic and secondary metabolite analysis of Aconitum violaceum Jacq.: a threatened medicinal herb. - Acta Physiol. Plant, 35: 2589-2599.
- SARMA P.P., GURUMAYUM N., VERMA A.K., DEVI, R., 2021 - A pharmacological perspective of banana: Implications relating to therapeutic benefits and molecular docking. - Food Funct., 12(11): 4749-4767.
- SINGH M.K., SHERPA A.R., HALLAN V., ZAIDI A.A., 2007 - A potyvirus in Cymbidium spp. in Northern India. - Australasian Plant Disease Notes, 2: 11-13.
- SINGH S.K., AGRAWALA D.K., JALAL J.S., DASH S.S., MAO A.A., SINGH P., 2019 - Orchids of India - A pictorial guide. - Botanical Survey of India, Kolkata, India, pp. 548.
- STEWART J., GRIFFITH M., 1995 - Manual of orchids. - Timber Press, Portland, Oregon, USA, pp. 370.
- SUDEEP R., RAJEEVAN P.K., VALASALAKUMARI P.K., GEETHA C.K., 1997 - Influence of organic supplements on shoot proliferation in Dendrobium. - J. Hortic. Navsari, 3: 38-44.
- TIKENDRA L., KOIJAM A.S., NONGDAM P., 2019 - Molecular markers based genetics fidelity assessment of micropropagated Dendrobium chrysotoxum Lindl. - Meta Gene, 20: 100562.
- VACIN E.F., WENT F.W., 1949 - Some pH changes in nutrient solutions. - Bot. Gaz., 110(4): 605-613.
- WILLIS K.J., 2017 - State of the World’s Plants. - Royal Botanical Gardens, Kew, London, UK, pp.
- WOCHOK Z.S., 1981 - The role of tissue culture in preserving threatened and endangered plant species. - Biol. Cons., 20(2): 83-89.
- XU Q., FU H., ZHU B., HASSAIN H.A., ZHANG K., TIAN X., DUAN M., XIE X., WANG L., 2021 - Potassium improves drought stress tolerance in plants by affecting root morphology, root exudates, and microbial diversity. - Metabolites, 11(3): 131.





