Published 2025-11-05
Keywords
- Callus formation,
- clonal propagation,
- Persea americana Mill.,
- root quality index
How to Cite
Copyright (c) 2025 Alejandro Facundo Barrientos-Priego, Mercedes Martínez-Villagómez , José Oscar Mascorro-Gallardo , Gabriel Iturriaga de la Fuente, María de la Cruz Espíndola-Barquera

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
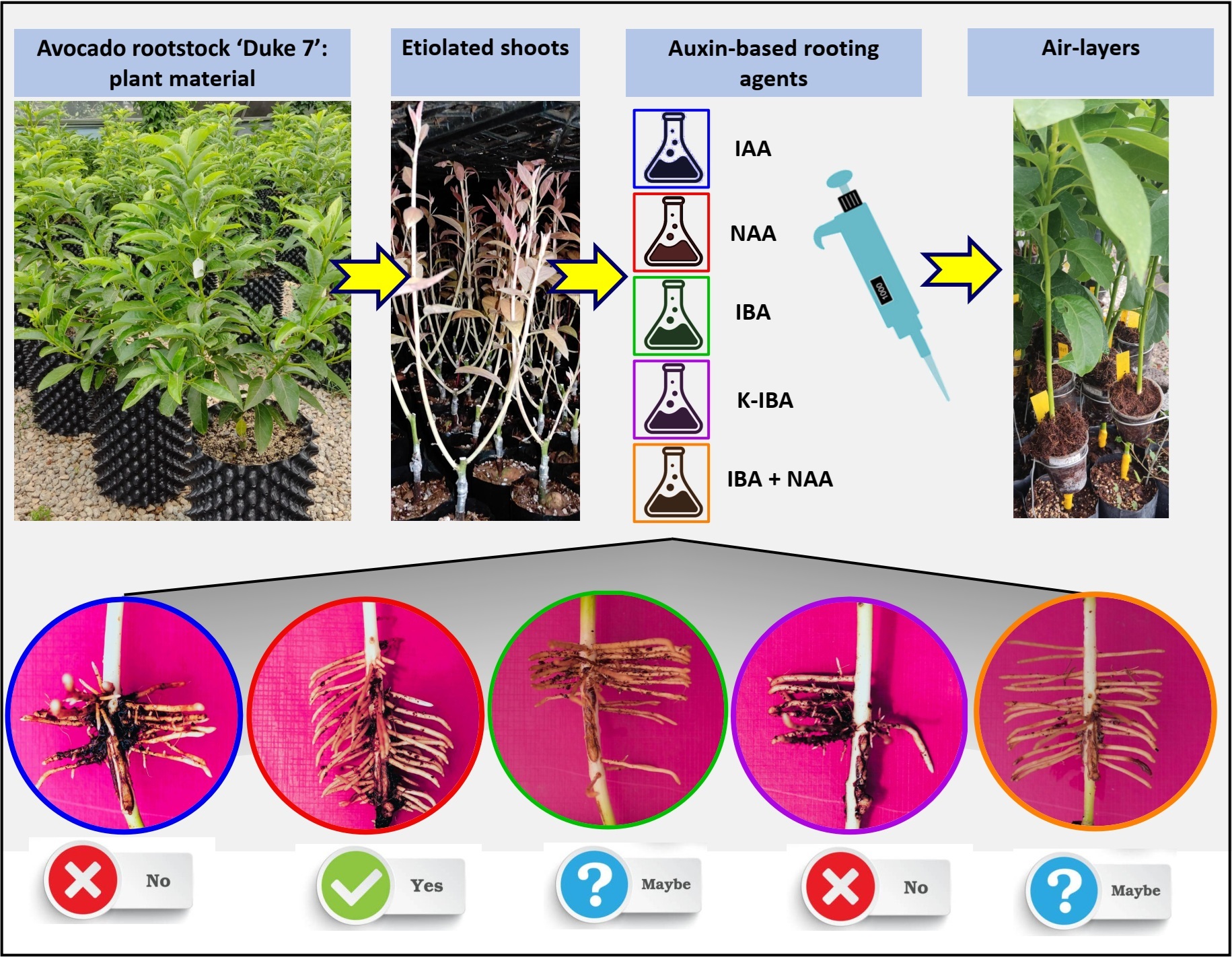
Clonal propagation of avocado rootstocks through etiolated shoot rooting represents a key strategy to enhance genetic uniformity, plant health, and productivity in commercial orchards. However, its success largely depends on the rooting phase, where auxins play a critical role. This study evaluated the effect of auxin-based rooting agents (types and concentrations) on root induction and quality in etiolated shoots of the ‘Duke 7’ rootstock. Five agents (IAA, NAA, IBA, K-IBA, and IBA + NAA combination) were tested at three concentrations (24.6, 34.4, and 44.2 mM) under a completely randomized factorial design (5 × 3) with three replicates per treatment. Morphological variables included rooting percentage, survival rate, root number/length/diameter, secondary root development, callus formation, and root quality index (RQI). Results revealed significant effects of agent type, concentration, and their interaction. NAA (34.4 mM) was the most effective for root number (55.3) and RQI (154.9 cm), albeit with high callus formation and reduced secondary roots. The IBA + NAA combination (34.4 mM) also showed high RQI (140.4 cm), with greater root length and less negative impact on root architecture. IBA alone achieved 100% rooting with moderate root development, balancing efficacy and physiological tolerance. Overall, intermediate concentrations of NAA and IBA + NAA yielded optimal results. These findings can refine clonal propagation protocols for ‘Duke 7’, with direct applications in commercial nurseries producing high-performance rootstocks.
References
- ALONI R., ALONI E., LANGHANS M., ULLRICH C.I., 2006 - Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. - Ann. Bot., 97(5): 883-893. DOI: https://doi.org/10.1093/aob/mcl027
- AMRI E., 2010 - Viable options and factors in consideration for low cost vegetative propagation of tropical trees. - Int. J. Bot., 6(2): 187-193. DOI: https://doi.org/10.3923/ijb.2010.187.193
- BARRIENTOS-PRIEGO A.F., 2017 - Presente y futuro de los portainjertos y variedades de aguacate en el mundo y México. - Proc. Fifth Lat. Am. Avocado Congr., pp. 2-15.
- BHALERAO R.P., EKLÖF J., LJUNG K., MARCHANT A., BENNETT M., SANDBERG G., 2002 - Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. - Plant J., 29(3): 325-332. DOI: https://doi.org/10.1046/j.0960-7412.2001.01217.x
- BISWAS M.S., FUKAKI H., MORI I.C., NAKAHARA K., MANO J., 2019 - Reactive oxygen species and reactive carbonyl species constitute a feed‐forward loop in auxin signaling for lateral root formation. - Plant J., 100(3): 536-548. DOI: https://doi.org/10.1111/tpj.14456
- CASANOVA-SÁEZ R., MATEO-BONMATÍ E., LJUNG K., 2021 - Auxin metabolism in plants. - Cold Spring Harb. Perspect. Biol., 13(3): a039867. DOI: https://doi.org/10.1101/cshperspect.a039867
- CASTRO M., FASSIO C., CRUZ R., 2021 - Efecto del tamaño de la semilla nodriza en el enraizamiento de paltos clonales. - Mem. VI Congr. Latinoam. Aguacate, pp. 1-6.
- CHEN W., HE L., TIAN S., MASABNI J., XIONG H., ZOU F., YUAN D., 2020 - Factors involved in the success of Castanea henryi stem cuttings in different cutting mediums and cutting selection periods. - J. For. Res., 32(4): 1627-1639. DOI: https://doi.org/10.1007/s11676-020-01208-5
- COHEN H., BAR-NOY Y., IRIHIMOVITCH V., RUBINOVICH L., 2023 - Effects of seedling and clonal West Indian rootstocks irrigated with recycled water on ‘Hass’ avocado yield, fruit weight and alternate bearing. - New Zealand J. Crop Hort. Sci., 51(1): 39-51. DOI: https://doi.org/10.1080/01140671.2022.2098779
- DA COSTA C.T., DE ALMEIDA M.R., RUEDELL C.M., SCHWAMBACH J., MARASCHIN F.S., FETT-NETO A.G., 2013 - When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. - Front. Plant Sci., 4: 133. DOI: https://doi.org/10.3389/fpls.2013.00133
- DA COSTA C.T., PEDEBOS C., VERLI H., FETT-NETO A.G., 2017 - The role of Zn2+, dimerization and N-glycosylation in the interaction of Auxin-Binding Protein 1 (ABP1) with different auxins. - Glycobiology, 27(12): 1109-1119. DOI: https://doi.org/10.1093/glycob/cwx080
- DAMODARAN S., STRADER L.C., 2019 - Indole 3-butyric acid metabolism and transport in Arabidopsis thaliana. - Front. Plant Sci., 10: 851. DOI: https://doi.org/10.3389/fpls.2019.00851
- DRUEGE U., FRANKEN P., HAJIREZAEI M.R., 2016 - Plant hormone homeostasis, signaling, and function during adventitious root formation in cuttings. - Front. Plant Sci., 7: 381. DOI: https://doi.org/10.3389/fpls.2016.00381
- DUMAN Z., HADAS-BRANDWEIN G., ELIYAHU A., BELAUSOV E., ABU-ABIED M., YESELSON Y., FAIGENBOIM A., LICHTER A., IRIHIMOVITCH V., SADOT E., 2020 - Short de-etiolation increases the rooting of VC801 avocado rootstock. - Plants, 9(11): 1481. DOI: https://doi.org/10.3390/plants9111481
- ERNST A., 1999 - Micro cloning: a multiple cloning technique for avocados using micro containers. - Rev. Chapingo Ser. Hortic., 5: 217-220.
- ERNST A.A., WHILEY A.W., BENDER G.S., 2013 - Propagation, pp. 234-267. - In: SCHAFFER B.A., B.N. WOLSTENHOLME, and A.W. WHILEY (eds.) The avocado: Botany, production and uses. CABI Int. Press, Wallingford, UK, pp. 416. DOI: https://doi.org/10.1079/9781845937010.0234
- ESCOBEDO V., ESCOBEDO J.A., 2011 - Adventitious root formation without rooting medium in etiolated shoots of ‘Duke’ avocado (Persea americana) growing on nurse plants. - Acta Horticulturae, 923: 227-232. DOI: https://doi.org/10.17660/ActaHortic.2011.923.34
- FETT-NETO A.G., FETT J.P., GOULART L.W.V., PASQUALI G., TERMIGNONI R.R., FERREIRA A.G., 2001 - Distinct effects of auxin and light on adventitious root development in Eucalyptus saligna and Eucalyptus globulus. - Tree Physiol., 21(7): 457-464. DOI: https://doi.org/10.1093/treephys/21.7.457
- FROLICH E.F., PLATT R.G., 1972 - Use of the etiolation technique in rooting avocado cuttings. - Calif. Avocado Soc. Yearb., 55: 97-109.
- GLEESON M., MITTER N., CARROLL B., 2016 - Etiolation-mediated regulation of adventitious rooting in avocado. - Acta Horticulturae, 1110: 35-40. DOI: https://doi.org/10.17660/ActaHortic.2016.1110.6
- GOMES G.L.B., SCORTECCI K.C., 2021 - Auxin and its role in plant development: structure, signalling, regulation and response mechanisms. - Plant Biol., 23(6): 894-904. DOI: https://doi.org/10.1111/plb.13303
- GROSSMANN K., 2009 - Auxin herbicides: current status of mechanism and mode of action. - Pest Manag. Sci., 66: 113-120. DOI: https://doi.org/10.1002/ps.1860
- HAMMES U.Z., MURPHY A.S., SCHWECHHEIMER C., 2021 - Auxin transporters - a biochemical view. - Cold Spring Harb. Perspect. Biol., 14(2): a039875. DOI: https://doi.org/10.1101/cshperspect.a039875
- HAYASHI K., ARAI K., AOI Y., TANAKA Y., HIRA H., GUO R., HU Y., GE C., ZHAO Y., KASAHARA H., FUKUI K., 2021 - The main oxidative inactivation pathway of the plant hormone auxin. - Nat. Commun., 12: 6752. DOI: https://doi.org/10.1038/s41467-021-27020-1
- HOFSHI R., 1996 - Experiments with cloning avocado rootstocks. - Calif. Avocado Soc. Yearb., 80: 103-108.
- JING H., STRADER L.C., 2019 - Interplay of auxin and cytokinin in lateral root development. - Int. J. Mol. Sci., 20(3): 486. DOI: https://doi.org/10.3390/ijms20030486
- KORASICK D.A., ENDERS T.A., STRADER L.C., 2013 - Auxin biosynthesis and storage forms. - J. Exp. Bot., 64(9): 2541-2555. DOI: https://doi.org/10.1093/jxb/ert080
- LAKEHAL A., BELLINI C., 2019 - Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. - Physiol. Plant., 165(1): 90-100. DOI: https://doi.org/10.1111/ppl.12823
- LESMES-VESGA R.A., CHAPARRO J.X., SARKHOSH A., RITENOUR M.A., CANO L.M., ROSSI L., 2021 - Effect of propagation systems and indole-3-butyric acid potassium salt (K-IBA) concentrations on the propagation of peach rootstocks by stem cuttings. - Plants, 10(6): 1151. DOI: https://doi.org/10.3390/plants10061151
- LI W., MA X., WANG S., HUANG W., JIANG M., 2024 - The leafy-stem-buried etiolation contributed to the high efficiency of rootstock vegetative propagation in avocado (Persea americana). - Horticulturae, 10(7): 770. DOI: https://doi.org/10.3390/horticulturae10070770
- LUDWIG-MÜLLER J., 2020 - Synthesis and hydrolysis of auxins and their conjugates with different side-chain lengths: are all products active auxins? - Period. Biol., 121-122(3-4): 81-96. DOI: https://doi.org/10.18054/pb.v121-122i3-4.10516
- MEDINA-URRUTIA V.M., BALTAZAR-LORENZO E., VIRGEN-CALLERO G., PIMIENTA-BARRIOS E., 2017 - Factores bióticos y abióticos que afectan la adaptación y crecimiento en plantaciones jóvenes de aguacate en Sayula, Jalisco, México. - Mem. V Congr. Latinoam. Aguacate, pp. 281-291.
- MELNYK C.W., 2016 - Plant grafting: insights into tissue regeneration. - Regen., 4(1): 3-14. DOI: https://doi.org/10.1002/reg2.71
- MINDÊLLO-NETO U.R., HIRANO E., TELLES C.A., BIASI L.A., 2006 - Propagação de abacateiro cv. Fuerte por estacas herbáceas. - Sci. Agrar., 7(1): 101. DOI: https://doi.org/10.5380/rsa.v7i1.7279
- NAPIER R., 2021 - The story of auxin-binding protein 1 (ABP1). - Cold Spring Harb. Perspect. Biol., 13: a039909. DOI: https://doi.org/10.1101/cshperspect.a039909
- NISSEN P., 1985 - Dose responses of auxins. - Physiol. Plant., 65(4): 357-374. DOI: https://doi.org/10.1111/j.1399-3054.1985.tb08659.x
- NISSEN S., SUTTER E., 1990 - Stability of IAA and IBA in nutrient medium to several tissue culture procedures. - HortSci., 25(7): 800-802. DOI: https://doi.org/10.21273/HORTSCI.25.7.800
- OCHOA-FUENTES Y.M., MARTÍNEZ-DE LA VEGA O., OLALDE-PORTUGAL V., CERNA-CHÁVEZ E., LANDEROS-FLORES J., HERNÁNDEZ-CASTILLO F.D., FLORES-OLIVAS A., 2007 - Genetic variability of Phytophthora cinnamomi Rands in Michoacan, Mexico. - Rev. Mex. Fitopatol., 25(2): 161-166.
- PINCELLI-SOUZA R.P., TANG Q., MILLER B.M., COHEN J.D., 2024 - Horticultural potential of chemical biology to improve adventitious rooting. - Hort. Adv., 2(12): 1-25. DOI: https://doi.org/10.1007/s44281-024-00034-7
- RAGGI S., DOYLE S.M., ROBERT S., 2020 - Auxin: at the crossroads between chemistry and biology, pp. 123-153. - In: GEELEN D., and L. XU (eds.) The chemical biology of plant biostimulants. John Wiley & Sons Ltd, Hoboken, USA, pp. 328. DOI: https://doi.org/10.1002/9781119357254.ch5
- ROGEL-CASTELLANOS I., MUÑOZ-PÉREZ R.B., CRUZ-CASTILLO J.G., 2000 - Propagación de aguacatero por acodo utilizando etiolación, ácido indolbutírico, y obstrucción de savia. - Rev. Chapingo Ser. Hortic., 6(1): 101-104. DOI: https://doi.org/10.5154/r.rchsh.1999.07.049
- ROUSSOS P.A., 2023 - Adventitious root formation in plants: the implication of hydrogen peroxide and nitric oxide. - Antioxidants, 2(4): 862-862. DOI: https://doi.org/10.3390/antiox12040862
- SAHOO G., SWAMY S.L., SINGH A.K., MISHRA A., 2021 - Propagation of Pongamia pinnata (L.) Pierre: Effect of auxins, age, season and C/N ratio on rooting of stem cuttings. - Trees For. People, 5: 100091. DOI: https://doi.org/10.1016/j.tfp.2021.100091
- SALAZAR-GARCÍA S., ROCHA-ARROYO J.L., IBARRA-ESTRADA M.E., BÁRCENAS-ORTEGA A.E., 2015 - Fenología de la raíz del aguacate ‘Hass’ en varios climas de Michoacán. - Proc. Eighth World Avocado Congr., pp. 277-283.
- SALAZAR-GARCÍA S., VELASCO-CÁRDENAS J.J., MEDINA-TORRES R., GÓMEZ-AGUILAR J.R., 2004 a - Selecciones de aguacate con potencial de uso como portainjertos. I. Prendimiento y crecimiento de injertos. - Rev. Fitotec. Mex., 27(1): 23-30. DOI: https://doi.org/10.35196/rfm.2004.1.23
- SALAZAR-GARCÍA S., VELASCO-CÁRDENAS J.J., MEDINA-TORRES R., GÓMEZ-AGUILAR J.R., 2004 b - Selecciones de aguacate con potencial de uso como portainjertos. II. Respuesta al enraizamiento mediante acodos. - Rev. Fitotec. Mex., 27(2): 183-190. DOI: https://doi.org/10.35196/rfm.2004.2.183
- SÁNCHEZ-GONZÁLEZ E.I., GUTIÉRREZ-SOTO J.G., OLIVARES-SÁENZ E., GUTIÉRREZ-DÍEZ A., BARRIENTOS-PRIEGO A.F., OCHOA-ASCENCIO S., 2019 - Screening progenies of Mexican race avocado genotypes for resistance to Phytophthora cinnamomi Rands. - HortScience, 54(5): 809-813. DOI: https://doi.org/10.21273/HORTSCI13552-18
- SOURATI R., SHARIFI P., POORGHASEMI M., ALVES V.E., SEIDAVI A., ANJUM N., SEHAR Z., and SOFO A., 2022 - Effects of naphthaleneacetic acid, indole-3-butyric acid and zinc sulfate on the rooting and growth of mulberry cuttings. - Int. J. Plant Biol., 13(3): 245-256. DOI: https://doi.org/10.3390/ijpb13030021
- TAHIR M.M., MAO J., LI S., LI K., LIU Y., SHAO Y., ZHANG D., ZHANG X., 2022 - Insights into factors controlling adventitious root formation in apples. - Horticulturae, 8(4): 276. DOI: https://doi.org/10.3390/horticulturae8040276
- WANG D., WANG G., SUN S., LU X., LIU Z., WANG L., TIAN W., LI Z., LI L., GAO Y., WANG K., 2024 - Research progress on cuttings of Malus rootstock resources in China. - Horticulturae, 10(3): 217. DOI: https://doi.org/10.3390/horticulturae10030217
- WANG J., JIN D., DENG Z., ZHENG L., GUO P., JI Y., SONG Z., ZENG H.Y., KINOSHITA T., LIAO Z., CHEN H., DENG X.W., WEI N., 2025 - The apoplastic pH is a key determinant in the hypocotyl growth response to auxin dosage and light. - Nat. Plants, 11: 279-294. DOI: https://doi.org/10.1038/s41477-025-01910-4
- YAN Y.-H., LI J.-L., ZHANG X.-Q., YANG W.-Y., WAN Y., MA Y.-M., ZHU Y.-Q., PENG Y., HUANG L.-K., 2014 - Effect of naphthalene acetic acid on adventitious root development and associated physiological changes in stem cutting of Hemarthria compressa. - PLoS ONE, 9(3): e90700. DOI: https://doi.org/10.1371/journal.pone.0090700
- YANG Y., HAMMES U.Z., TAYLOR C.G., SCHACHTMAN D.P., NIELSEN E., 2006 - High-affinity auxin transport by the AUX1 influx carrier protein. - Curr. Biol., 16(11): 1123-1127. DOI: https://doi.org/10.1016/j.cub.2006.04.029
- YANG Y., LIU X., GUO W., LIU W., SHAO W., ZHAO J., LI J., DONG Q., MA L., HE Q., LI Y., HAN J., and LEI X., 2022 - Testing the polar auxin transport model with a selective plasma membrane H+‐ATPase inhibitor. - J. Integr. Plant Biol., 64(6): 1229-1245. DOI: https://doi.org/10.1111/jipb.13256
- YUN F., LIU H., DENG Y., HOU X., LIAO W., 2023 - The role of light-regulated auxin signaling in root development. - Int. J. Mol. Sci., 24(6): 5253. DOI: https://doi.org/10.3390/ijms24065253
- ZHAI R., XU L., 2021 - Pluripotency acquisition in the middle cell layer of callus is required for organ regeneration. - Nat. Plants, 7(11): 1453-1460. DOI: https://doi.org/10.1038/s41477-021-01015-8
- ZHANG Y., BERMAN A., SHANI E., 2023 - Plant hormone transport and localization: signaling molecules on the move. - Annu. Rev. Plant Biol., 74(1): 453-479. DOI: https://doi.org/10.1146/annurev-arplant-070722-015329
- ZHANG Y., LI J., LI C., CHEN S., TANG Q., XIAO Y., ZHONG L., CHEN Y., CHEN B., 2022 - Gene expression programs during callus development in tissue culture of two Eucalyptus species. - BMC Plant Biol., 22(1): 1-18. DOI: https://doi.org/10.1186/s12870-021-03391-x
- ZHAO Y., CHEN Y., JIANG C., LU M.-Z., ZHANG J., 2022 - Exogenous hormones supplementation improve adventitious root formation in woody plants. - Front. Bioeng. Biotechnol., 10: 1009531. DOI: https://doi.org/10.3389/fbioe.2022.1009531





